Douglas has a robust portfolio of innovative projects in various therapeutic areas. We’re seeking co-development partners with complementary expertise and resources to accelerate the development and commercialisation of these promising therapies.
Our pipelines
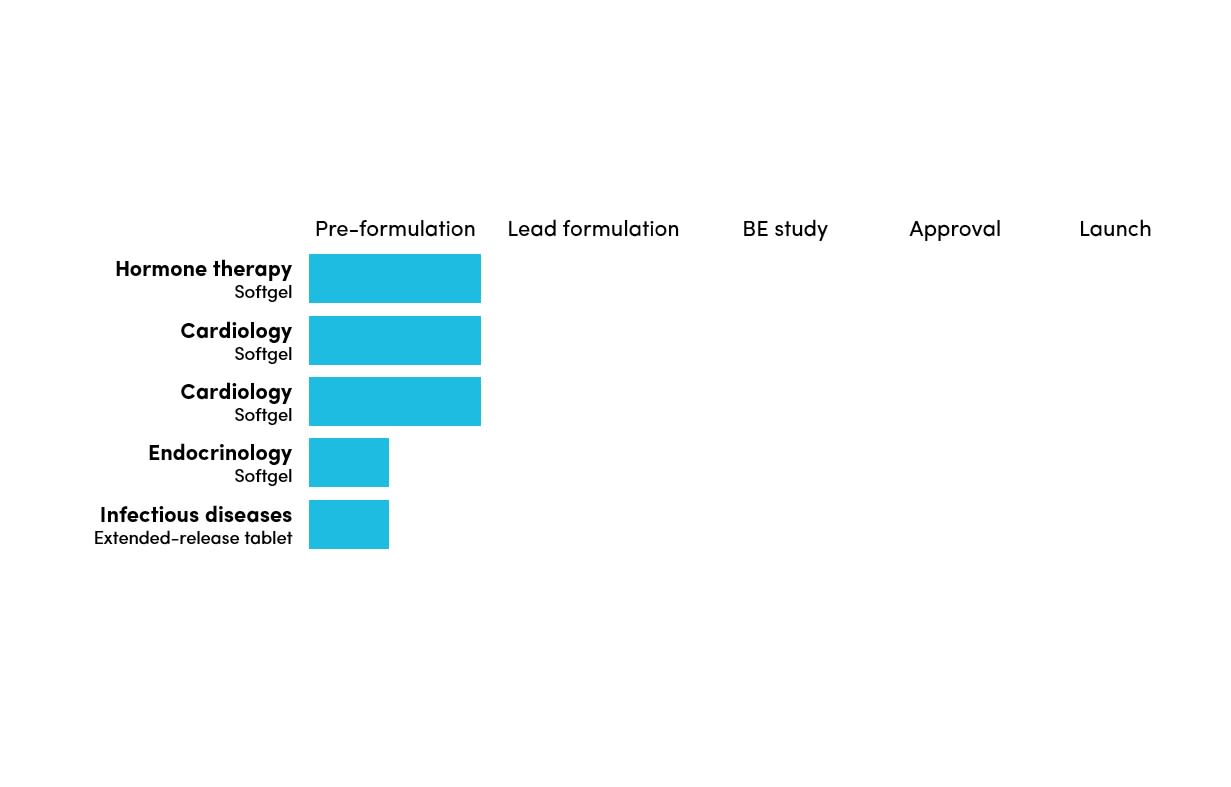
505(j) Complex Generic Pipeline
Douglas has several projects in the pre-formulation or formulation development stage.
Douglas specialises in specialty generic product development such as soft-gelatin capsules that have technical, clinical or IP complexity.
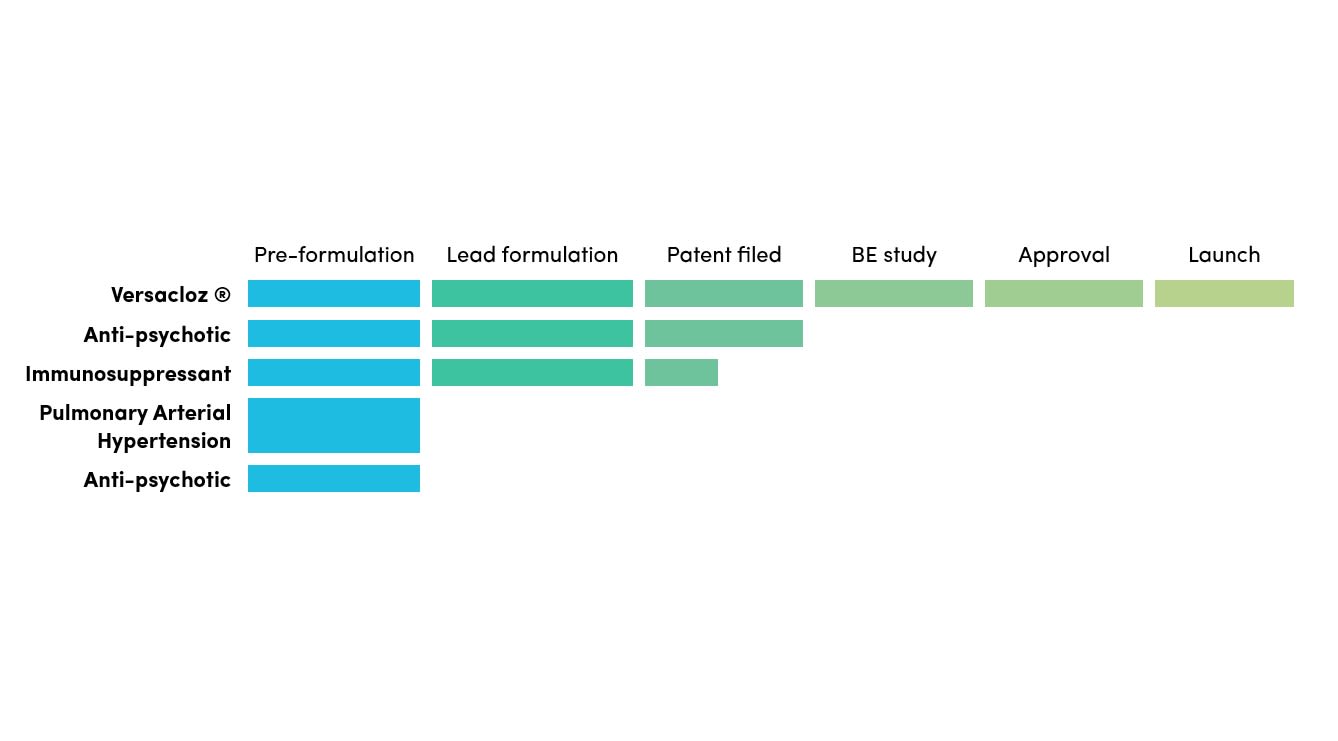
505(b)(2) Pipeline
Douglas specialises in the development of 505(b)(2) alternative formulation products to meet unmet needs in populations of paediatric, geriatric and patients with swallowing difficulties.
We are open to collaboration on these oral liquid opportunities focusing on the US market.